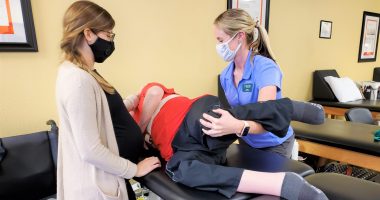
Using Dry Needling To Relieve Muscle Spasms For SMA Patients
Dry needling has been a popular treatment in the world of physical therapy for the past few years and is continuing to gain popularity with more research being performed. There are many different reasons to use dry needling to improve your patients’ function, with the main reason being to relax…