Long-term Spinraza found safe, effective across SMA spectrum
Long-term treatment with Spinraza (nusinersen) was associated with improved or stable motor function in patients across the spinal muscular atrophy (SMA) disease spectrum, according to an analysis of real-world registry data.
“To date, this is the largest prospective study over the longest observational period of [Spinraza] therapy in adult patients with SMA,” researchers wrote.
“Our results demonstrate sustained efficacy across the phenotypic [disease characteristics] spectrum of adult SMA, showing stabilisation or improvement in motor function in a large number of patients, which is clearly different from the natural history of the disease.”
The study, “Long-term efficacy and safety of nusinersen in adults with 5q spinal muscular atrophy: a prospective European multinational observational study,” was published in The Lancet Regional Health – Europe.
Spinraza was first approved disease-modifying therapy for SMA
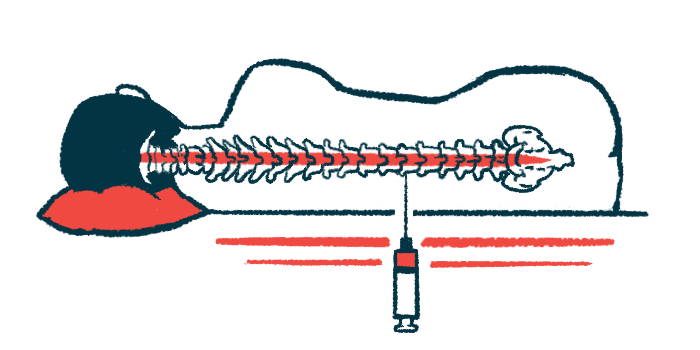
Biogen’s Spinraza was the first disease-modifying therapy for SMA to be approved, receiving regulatory clearance in the U.S. in 2016 and Europe in 2017. Delivered directly into the spinal canal — called an intrathecal injection or lumbar puncture — the medication is designed to increase levels of the SMN protein that SMA patients lack.
While its efficacy has been well-established in clinical trials, long-term, real-world data involving a wide range of SMA patients with varying degrees of disease severity are still needed, according to the researchers.
As such, the researchers examined long-term outcomes from 237 SMA patients who were treated with Spinraza in Europe, including individuals across all SMA types, different walking abilities, and a wide age range.
The data came from SMArtCARE, a registry that collects information from SMA patients in Germany, Switzerland, and Austria. It’s supported by the companies that market the three available SMA therapies: Novartis, which markets gene therapy Zolgensma, Roche, marketer of Evrysdi, and Biogen.
Patients ranged in age from 16 to 71, with a mean of 36 years. Most had SMA type 3 (66%) or type 2 (28%). Under half (42%) retained their ability to walk (ambulatory).
All were treated with Spinraza for at least 14 months, or a little over a year, in accordance with the therapy’s prescribing label.
Study evaluated changes in motor function with Spinraza treatment
The study’s main goal was to evaluate changes in motor function, as assessed by the Hammersmith Functional Motor Scale Expanded (HFMSE), in patients while on Spinraza compared to pre-treatment (baseline).
HFMSE performance was collected at baseline, and after 14 months (237 patients), 26 months (a little over two years; 171 patients), and 38 months (a little over three years; 120 patients) on Spinraza.
Mean HFMSE scores were significantly higher after 14, 26, and 36 months on Spinraza compared with baseline.
Clinically meaningful improvements, defined as an increase of at least 3 points on the HFMSE, were observed in 29%-30% of patients at each time point on Spinraza. Of 68 people who saw a clinically meaningful improvement after 14 months, 28 maintained it through month 38.
Consistent with the primary analysis, mean scores on the Revised Upper Limb Module (RULM) and distance walked on the six-minute walk test (6MWT) were significantly increased at all time points after initiating Spinraza. RULM is a measure of upper limb motor function and 6MWT is an indicator of exercise capacity.
Exploratory analyses generally demonstrated motor improvements assessed by HFMSE were greater in less severely affected patients, that is, those with SMA type 3, who were ambulatory, and who had not undergone spinal fusion surgery.
In more severely affected patients (i.e. SMA type 2; non-ambulatory), the clinical improvements were smaller, “however, we did observe sustained stabilisation of symptoms,” the researchers wrote, noting that previous natural history studies indicate a decline in motor function would be expected for those patients if left untreated.
Stabilization of disease can be interpreted as treatment efficacy: Researchers
“Stabilisation of the otherwise progressive disease course in severely affected patients can be interpreted as efficacy,” the team wrote.
On the other hand, RULM appeared more sensitive for capturing changes in more severely affected patients. There, those with SMA type 2 and non-ambulatory patients saw greater changes from baseline than less severely affected patients.
Such findings highlight an important caveat to the use of certain motor function scales in severely and mildly affected patients, according to the scientists.
For example, the HFMSE may be subject to so-called floor effects in severely affected patients, where performance is too close to the minimum score to assess meaningful changes over time.
The opposite may be true for RULM, where less severely affected patients may be subject to a ceiling effect, according to the scientists.
Still, “as no biomarker of therapeutic response to [Spinraza] has yet been validated for adult patients in clinical routine, clinical scales remain the most relevant outcome parameter to date,” the team wrote, noting a composite of the two measures may be of benefit.
No new safety signals were identified, with the most common adverse events being post-lumbar puncture syndrome (a complication that can follow intrathecal injections), headache, back pain, and infections.
The post Long-term Spinraza found safe, effective across SMA spectrum appeared first on SMA News Today.






