Target the genetic root cause of the disease



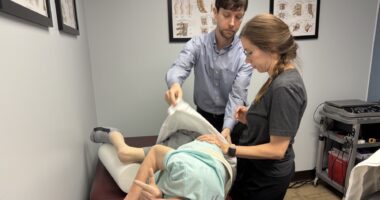
Any parent who has received a spinal muscular atrophy (SMA) diagnosis for their child can tell you that early treatment is crucial. Children who receive treatment for SMA as soon as possible are more likely to achieve important motor milestones. One-time gene therapy ZOLGENSMA® (onasemnogene abeparvovec-xioi) can stop the progression of SMA.

“From the beginning, I wanted ZOLGENSMA because it was a gene therapy and it was a one-time treatment.”
Ciji, mother of Maisie who has SMA Type 1
ZOLGENSMA is a prescription gene therapy used to treat children less than 2 years old with SMA. ZOLGENSMA has a risk of acute serious liver injury and acute liver failure, and in clinical trials the most common side effects were elevated liver enzymes and vomiting. Please keep reading for additional Important Safety Information and please see the Full Prescribing Information.
How does a one-time-only dose of ZOLGENSMA work to stop the progression of SMA?
In children with SMA, the SMN1 gene is missing or not working properly. This gene provides instructions for the motor neuron cells to produce survival motor neuron (SMN) protein. Motor neurons are responsible for all types of muscle function. So when the SMN1 gene is missing or not working and motor neuron cells don’t have enough SMN protein to survive, muscles become weak. Once motor neuron cells stop working, they cannot be brought back. This means basic muscle functions become harder to do and can be lost permanently. In SMA, the body relies on the SMN2 backup gene; however, 90% of the SMN protein that the SMN2 backup gene makes is not fully functional. Usually, the more copies of the SMN2 gene a person has, the less severe his or her SMA is. So how do we solve this problem?
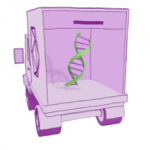
ZOLGENSMA is a gene therapy that targets the genetic root cause of SMA with a one-time-dose. The main components of ZOLGENSMA are a gene and a vector. A new, working SMN gene is placed inside the vector which acts like a delivery truck, bringing the SMN gene to motor neuron cells throughout the body.
ZOLGENSMA replaces the function of the SMN1 gene to make SMN protein with a one-time dose. With the motor neuron cells now able to make sufficient SMN protein, motor neuron cells that have not died may survive, function, and be maintained. This means muscles can function to help children with SMA achieve motor milestones that were once thought impossible without treatment.
Results and outcomes vary among children based on several factors, including how far their SMA symptoms progressed prior to receiving treatment.
Ask your doctor if ZOLGENSMA could be right for your child with SMA.
To learn more, visit ZOLGENSMA.com.
Indication and Important Safety Information
What is ZOLGENSMA?
ZOLGENSMA is a prescription gene therapy used to treat children less than 2 years old with spinal muscular atrophy (SMA). ZOLGENSMA is given as a one-time infusion into a vein. ZOLGENSMA was not evaluated in patients with advanced SMA.
What is the most important information I should know about ZOLGENSMA?
- ZOLGENSMA can increase liver enzyme levels and cause acute serious liver injury or acute liver failure.
- Patients will receive an oral corticosteroid before and after infusion with ZOLGENSMA and will undergo regular blood tests to monitor liver function.
- Contact the patient’s doctor immediately if the patient’s skin and/or whites of the eyes appear yellowish, if the patient misses a dose of corticosteroid or vomits it up, or if the patient experiences a decrease in alertness.
What should I watch for before and after infusion with ZOLGENSMA?
- Infections before or after ZOLGENSMA infusion can lead to more serious complications. Contact the patient’s doctor immediately if you see any signs of a possible infection such as coughing, wheezing, sneezing, runny nose, sore throat, or fever.
- Decreased platelet counts could occur following infusion with ZOLGENSMA. Seek immediate medical attention if the patient experiences unexpected bleeding or bruising.
- Thrombotic microangiopathy (TMA) has been reported to occur approximately one week after ZOLGENSMA infusion. Caregivers should seek immediate medical attention if the patient experiences any signs or symptoms of TMA, such as unexpected bruising or bleeding, seizures, or decreased urine output.
What do I need to know about vaccinations and ZOLGENSMA?
- Talk with the patient’s doctor to decide if adjustments to the vaccination schedule are needed to accommodate treatment with a corticosteroid.
- Protection against respiratory syncytial virus (RSV) is recommended.
Do I need to take precautions with the patient’s bodily waste?
Temporarily, small amounts of ZOLGENSMA may be found in the patient’s stool. Use good hand hygiene when coming into direct contact with bodily waste for 1 month after infusion with ZOLGENSMA. Disposable diapers should be sealed in disposable trash bags and thrown out with regular trash.
What are the possible or likely side effects of ZOLGENSMA?
The most common side effects that occurred in patients treated with ZOLGENSMA were elevated liver enzymes and vomiting.
The safety information provided here is not comprehensive. Talk to the patient’s doctor about any side effects that bother the patient or that don’t go away.
You are encouraged to report suspected side effects by contacting the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch, or Novartis Gene Therapies, Inc. at 833-828-3947.
Please see the Full Prescribing Information.
The preceding article is content provided by our sponsor, Novartis Gene Therapies, Inc. The views and opinions expressed in the content above are not the views and opinions of SMA News Today or its parent company, BioNews, Inc.
SMA News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
©2022 Novartis Gene Therapies, Inc. Bannockburn, IL 60015 10/2022 US-ZOL-22-0162
The post Target the genetic root cause of the disease appeared first on SMA News Today.