SMA News, Late January Edition: Serum Creatinine Biomarkers, Intrathecal Nusinersen & More

New SMA research and news related to newborn screening and the upcoming FDA evaluation of a new drug have kicked off 2020. Below is a roundup of the latest on SMA.
Understanding SMA
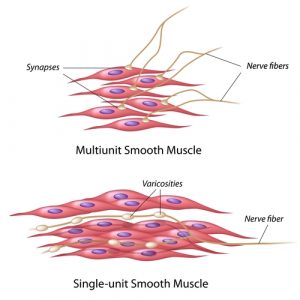 Serum creatinine is a biomarker of progressive denervation in spinal muscular atrophy.1
Serum creatinine is a biomarker of progressive denervation in spinal muscular atrophy.1
This paper describes a study into the notion that serum creatinine could be a valuable prognostic biomarker to monitor denervation progression in SMA patients. The researchers examined data from 238 patients with SMA and found that creatinine levels were related to SMA disease severity. Specifically, decreased creatinine concentrations were associated with more severe forms of the disease. Based on these observations, the authors conclude that creatinine measurements should be part of routine analyses for patients with SMA. They also point to the need to determine if creatinine levels change in response to SMA therapies.
Read more here.
Resolution of pathogenic R-loops rescues motor neuron degeneration in spinal muscular atrophy.2
This commentary in response to ‘ZPR1 prevents R-loop accumulation, upregulates SMN2 expression, and rescues spinal muscular atrophy’ argues that crosstalk that occurs between DNA damage response and cell death pathways may contribute to the pathogenesis of SMA.
Read more here.
Genomic analysis of a spinal muscular atrophy (SMA) discordant family identifies a novel mutation in TLL2, an activator of growth differentiation factor 8 (myostatin): a case report.3
This case report identifies new genetic modifiers of SMA using whole exome sequencing in the context of an SMA discordant family. The authors argue that this new information will improve the accuracy of the counseling that is provided to SMA patients and their families.
Read more here.
Limitations of six-minute Walk Test Reference Values for Spinal Muscular Atrophy.4
This paper describes an investigation into the reliability of the six-minute walk test (6MWT) as a measure of walking ability in SMA. According to the authors, new disease-modifying therapies for SMA necessitate normative reference data that allow for comparisons between healthy controls and patients with SMA. Their review of the literature demonstrated result variability in 6MWT, pointing to a potential limitation in the use of this test.
Read more here.
Treating SMA
 Intrathecal nusinersen treatment after ventriculo-peritoneal shunt placement: A case report focusing on the neurofilament light chain in cerebrospinal fluid.5
Intrathecal nusinersen treatment after ventriculo-peritoneal shunt placement: A case report focusing on the neurofilament light chain in cerebrospinal fluid.5
This case report describes a female SMA patient who developed progressive hydrocephalus in infancy and had a ventriculo-peritoneal shunt implanted. When she was 7 years old, nusinersen treatment was initiated, and treatment was associated with a reduction in the concentration of neurofilament light-chain in her cerebrospinal fluid.
In July 2018, communicating hydrocephalus was reported in 5 patients undergoing nusinersen treatment, some of whom were managed with a ventriculo-peritoneal shunt. There has since been great interest in the safety of nusinersen treatment in patients with this type of shunt. The authors have therefore provided this report to demonstrate that nusinersen may be safe – as well as effective – in those with ventriculo-peritoneal shunts. Based on their results, they also point to the possibility that neurofilament light chain could be a valuable SMA marker.
Read more here.
Cerebrospinal fluid proteomic profiling in nusinersen-treated patients with spinal muscular atrophy.6
In this piece, the authors describe their analysis of cerebrospinal fluid proteomic profiles in MA patients and healthy controls. They identified relevant differences in the profiles of SMA patients and controls, as well as differences between nusinersen-treated SMA patients who did and who did not respond to treatment. Based on their observations, the authors conclude that this type of comparative analysis in adult SMA patients could be a valuable diagnostic and predictive tool.
Read more here.
Drosophila SMN2 minigene reporter model identifies moxifloxacin as a candidate therapy for SMA.7
This piece describes how a Drosophila model was used to screen potential SMA drugs and how it led to the discovery of a small molecule that could potentially treat SMA. The molecule, moxifloxacin, has been approved by the FDA.
Read more here.
Recent Review:
- Expanding therapeutic opportunities for neurodegenerative diseases: A perspective on the important role of phenotypic screening.8
This review highlights the lack of new FDA-approved drugs for neurodegenerative diseases and points to the development of non-antisense oligonucleotide splice modulators for SMA. According to the authors, the phenotypic screening approach used to identify these molecules may be a valuable way to uncover new drug targets for other neurodegenerative diseases.
Read more here.
- Spinal muscular atrophy – new therapies, new challenges.9
In this review, new insights regarding SMA are discussed, as are the newest therapies for the disease. The authors highlight progress in the therapeutic domain of the disease and raise important questions related to the administration of these therapies and the implications for newborn screening.
Read more here.
SMA Management
 Ultra-high-frequency ultrasound in patients with spinal muscular atrophy: A retrospective feasibility study.10
Ultra-high-frequency ultrasound in patients with spinal muscular atrophy: A retrospective feasibility study.10
In this letter to the editor, the authors describe their findings supporting the use of ultra-high frequency ultrasound in children with SMA. Specifically, they claim that this technique is an easy, non-invasive way to image peripheral nerves to identify and visualize novel features in high resolution.
Read more here.
The value of preoperative labs in identifying “at-risk” patients for developing surgical site infections after pediatric neuromuscular spine deformity surgery.11
This retrospective cohort study aimed to determine if current methods for evaluating risk for surgical site infection in patients preparing to undergo posterior spinal fusion are appropriate and useful. The authors found that in the case of pediatric neuromuscular scoliosis, preoperative nutritional labs, including Hgb/Hct and TLC values did not correlate with surgical site infection risk and therefore call into question the utility of conducting these tests in this context.
Read more here.
Case of spinal muscular atrophy type 0 with mild prognosis.12
This case report describes a baby boy with SMA type 0 that responded to nusinersen therapy. Given that SMA type 0 is generally associated with poor prognoses, the authors interpret the response to therapy to mean that there is a mild form of SMA type 0 that can occur at birth.
Read more here.
SMA News
- Senator Robert Hilkemann of Nebraska has introduced a new bill that would require that SMA be added to the 32 core conditions that newborns are currently screened for in that state. Read more here.13
- Senator Troy Singleton of New Jersey has sponsored legislation requirement newborn infants in his state to be screened for SMA. The bill has passed the Senate and would require the Commissioner of Health to create a comprehensive program for follow-up services in the cases of positive tests for SMA. See more details of the bill here.14
- Connecticut will begin testing newborns for SMA this month. Their state legislature passed this requirement last year. Read more here.15
- Though no specific pricing plans have been released, it has been reported that the drug maker Roche plans to competitively price its new oral SMA drug, risdiplam. The FDA should make a decision on the drug in May. Read more here.16
References
1. Alves CRR, Zhang R, Johnstone AJ, et al. Serum creatinine is a biomarker of progressive denervation in spinal muscular atrophy. Neurology. December 2019. doi:10.1212/WNL.0000000000008762
2. Hensel N, Detering NT, Walter LM, Claus P. Resolution of pathogenic R-loops rescues motor neuron degeneration in spinal muscular atrophy. Brain. 2020;143(1):2-5. doi:10.1093/brain/awz394
3. Jiang J, Huang J, Gu J, Cai X, Zhao H, Lu H. Genomic analysis of a spinal muscular atrophy (SMA) discordant family identifies a novel mutation in TLL2, an activator of growth differentiation factor 8 (myostatin): a case report. BMC Med Genet. 2019;20(1):204. doi:10.1186/s12881-019-0935-3
4. Goodwin AM, Cornett KMD, McKay MJ, et al. Limitations of six-minute Walk Test Reference Values for Spinal Muscular Atrophy. Muscle Nerve. December 2019. doi:10.1002/mus.26794
5. Tozawa T, Kasai T, Tatebe H, et al. Intrathecal nusinersen treatment after ventriculo-peritoneal shunt placement: A case report focusing on the neurofilament light chain in cerebrospinal fluid. Brain Dev. December 2019. doi:10.1016/j.braindev.2019.12.006
6. Kessler T, Latzer P, Schmid D, et al. Cerebrospinal fluid proteomic profiling in nusinersen-treated patients with spinal muscular atrophy. J Neurochem. January 2020:e14953. doi:10.1111/jnc.14953
7. Konieczny P, Artero R. Drosophila SMN2 minigene reporter model identifies moxifloxacin as a candidate therapy for SMA. FASEB J Off Publ Fed Am Soc Exp Biol. December 2019. doi:10.1096/fj.201802554RRR
8. Swalley SE. Expanding therapeutic opportunities for neurodegenerative diseases: A perspective on the important role of phenotypic screening. Bioorg Med Chem. December 2019:115239. doi:10.1016/j.bmc.2019.115239
9. Jedrzejowska M, Kostera-Pruszczyk A. Spinal muscular atrophy – new therapies, new challenges. Neurol Neurochir Pol. January 2020. doi:10.5603/PJNNS.a2019.0068
10. Regensburger AP, Wagner AL, Hanslik G, et al. Ultra-high-frequency ultrasound in patients with spinal muscular atrophy: A retrospective feasibility study. Muscle Nerve. December 2019. doi:10.1002/mus.26796
11. Furdock R, Luhmann SJ. The value of preoperative labs in identifying “at-risk” patients for developing surgical site infections after pediatric neuromuscular spine deformity surgery. Spine Deform. January 2020. doi:10.1007/s43390-019-00003-5
12. Kitaoka H, Shitara Y, Uchida Y, Kondo U, Omori I. Case of spinal muscular atrophy type 0 with mild prognosis. Pediatr Int. January 2020. doi:10.1111/ped.14047
13. Austin J. Bill would add spinal muscular atrophy to infant screening panel. 1011 Now. https://www.1011now.com/content/news/Bill-would-add-Spinal-Muscular-Atrophy-to-infant-screening-panel-566881771.html. Accessed January 15, 2020.
14. Troy Singleton – (D) New Jersey. Bill Track 50. https://www.billtrack50.com/LegislatorDetail/16982. Accessed January 16, 2020.
15. Dunavin D. New spinal muscular atrophy screening for Connecticut infants. NPR. https://www.wshu.org/post/new-spinal-muscular-atrophy-screening-connecticut-infants#stream/0. Published 2020. Accessed January 15, 2020.
16. Roche aims to “underwhelm” on SMA drug price to challenge rivals. Reuters. https://www.reuters.com/article/us-roche-sma/roche-aims-to-underwhelm-on-sma-drug-price-to-challenge-rivals-idUSKBN1ZD12F. Published 2020. Accessed January 15, 2020.

